How it Works
Leukocyte Function Associated Antigen-1 (LFA-1) plays a key role in extravasation, which is the process by which leukocytes leave the bloodstream to enter the tissues. PANOQUELL®-CA1 (fuzapladib sodium for injection) is a LFA-1 activation inhibitor.1
LFA-1 Inflammatory Pathway
When injured, the pancreas releases cytokines. Activation of LFA-1 occurs. This initiates the cascade of events which leads to neutrophilic extravasation into the pancreatic tissue.
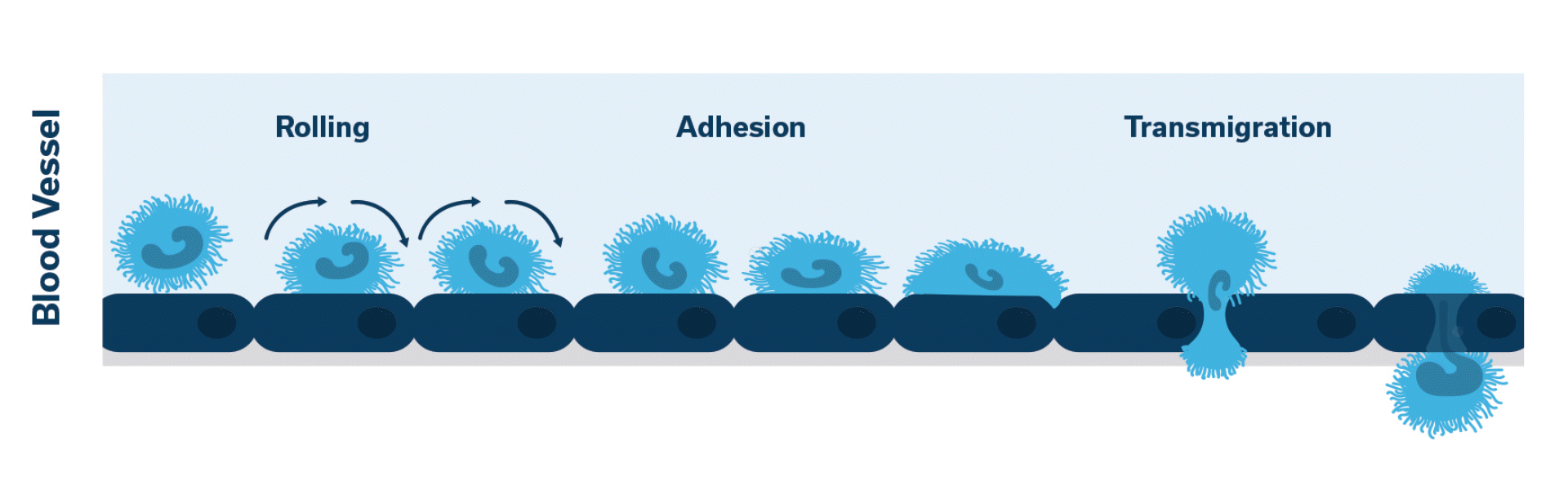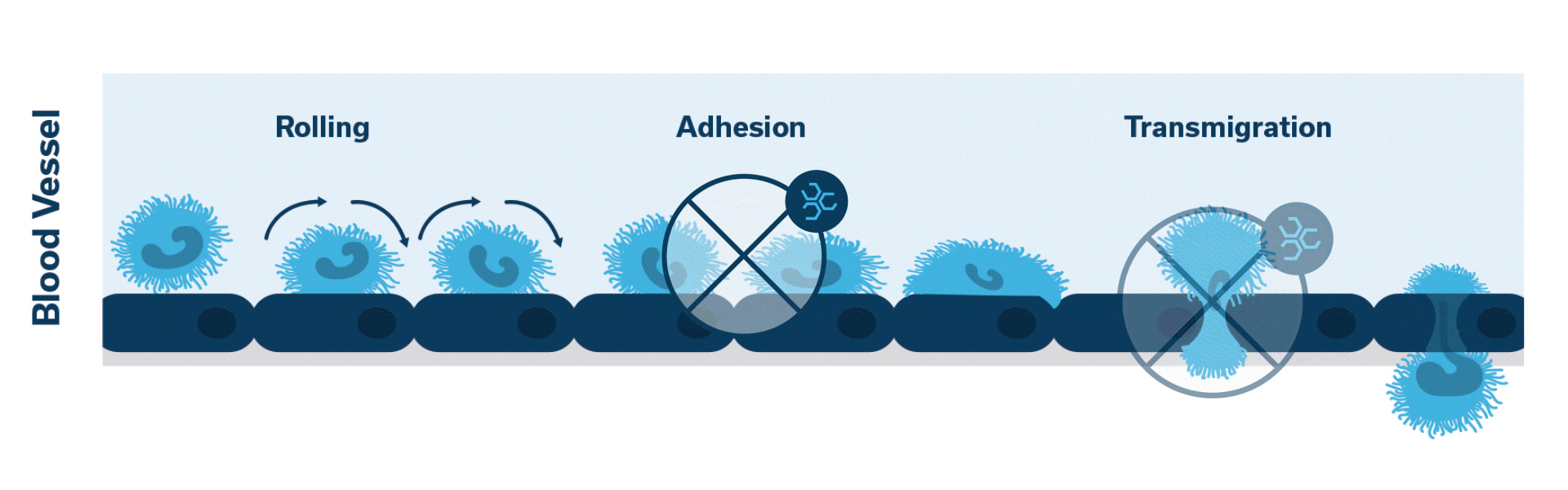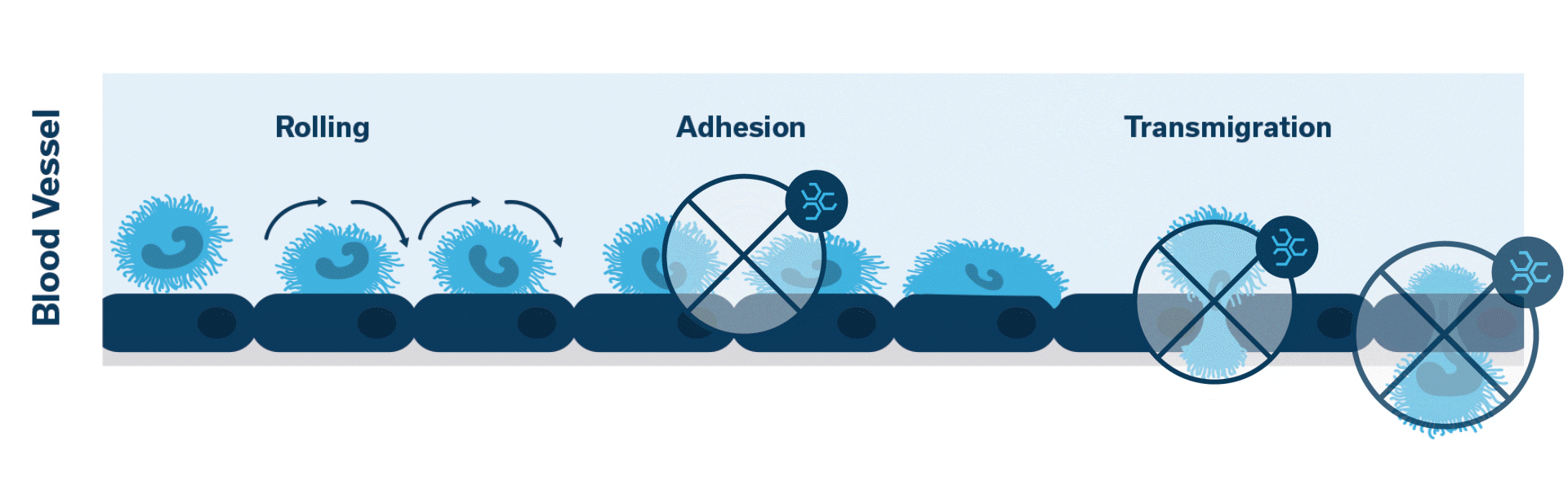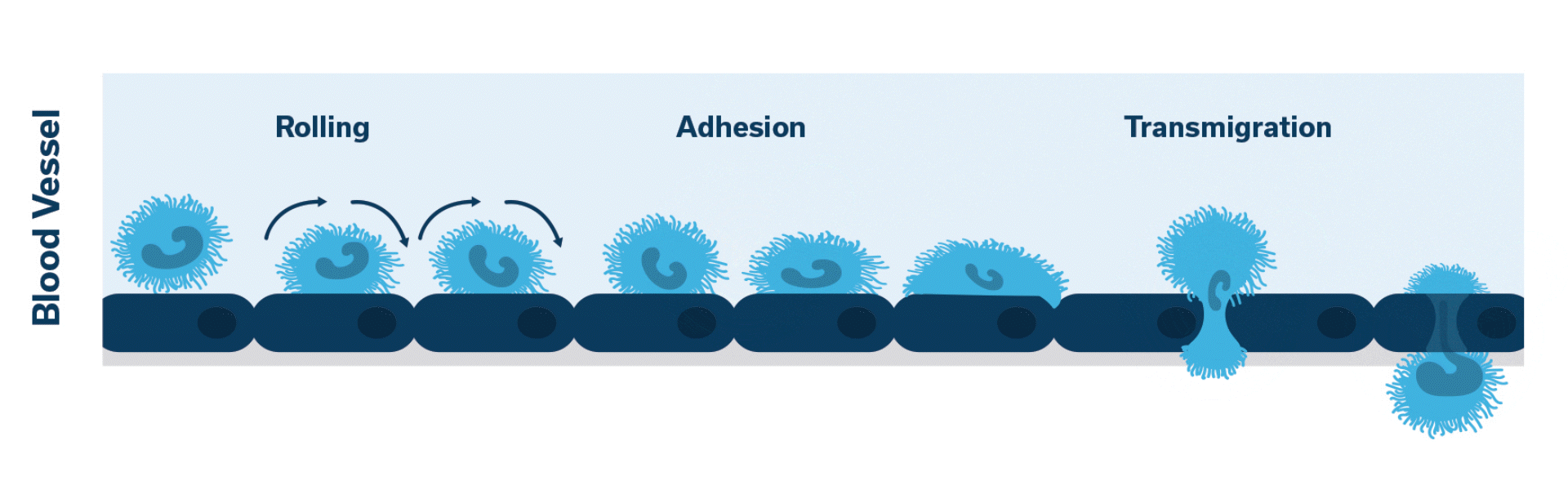

Once activated, neutrophils (leukocytes) begin to stick, roll along the vascular endothelium and eventually adhere and flatten to prepare for transmigration.
Extravasation of neutrophils into the pancreatic tissue is the hallmark of acute canine pancreatitis.
Once activated, neutrophils (leukocytes) begin to stick, roll along the vascular endothelium and eventually adhere and flatten to prepare for transmigration.
Extravasation of neutrophils into the pancreatic tissue is the hallmark of acute canine pancreatitis.
Watch our video on Extravasation of Neutrophils to learn more!
Watch our video on Extravasation of Neutrophils to learn more!
Administration
PANOQUELL®-CA1 is 4 mg/mL solution when reconstituted. It comes in a multi-use vial. Once reconstituted, it remains stable under refrigeration for 28 days.
PANOQUELL®-CA1 consists of two separate vials.
One vial contains 14 mg of fuzapladib sodium, 52.5 mg of D-mannitol, and 21 mg of tromethamine as sterile lyophilized powder. The second vial of 3.9 mL sterile diluent (bacteriostatic water for injection), containing 1.8% w/v benzyl alcohol, is for reconstituting the sterile lyophilized powder prior to use.
No other diluent should be used.
PANOQUELL®-CA1 is an intravenous (IV) injection dosed once a day for 3 days. The IV injection can be given over 15 seconds to 1 minute as a bolus.
Steps for reconstitution:

Using a sterile needle and syringe, withdraw 3.5 mL of the sterile diluent from the vial and slowly transfer the sterile diluent into the vial containing the sterile PANOQUELL®-CA1 lyophilized powder through the stopper. There is more sterile diluent supplied than the 3.5 mL needed for reconstitution.

Once the sterile diluent has been added to the powder vial, remove the needle and syringe from the vial. Discard unused sterile diluent, syringe, and needle.

Gently swirl the vial until the powder is fully reconstituted into solution, leaving no visible residue or un-dissolved material.

Before each use, gently swirl to ensure a uniform solution.

Draw up the appropriate dose using a new sterile needle and syringe.

Administer the dose promptly after drawing into the dosing syringe.

Store any remaining reconstituted product at refrigerated conditions, 36˚ to 46˚F (2˚ to 8˚C). Reconstituted product remains stable under refrigeration for 28 days.
Refer to product insert for more information.
Administration
PANOQUELL®-CA1 is 4 mg/mL solution when reconstituted. It comes in a multi-use vial. Once reconstituted, it remains stable under refrigeration for 28 days.
PANOQUELL®-CA1 consists of two separate vials.
One vial contains 14 mg of fuzapladib sodium, 52.5 mg of D-mannitol, and 21 mg of tromethamine as sterile lyophilized powder. The second vial of 3.9 mL sterile diluent (bacteriostatic water for injection), containing 1.8% w/v benzyl alcohol, is for reconstituting the sterile lyophilized powder prior to use.
No other diluent should be used.
PANOQUELL®-CA1 is an intravenous (IV) injection dosed once a day for 3 days. The IV injection can be given over 15 seconds to 1 minute as a bolus.
Steps for reconstitution:

Using a sterile needle and syringe, withdraw 3.5 mL of the sterile diluent from the vial and slowly transfer the sterile diluent into the vial containing the sterile PANOQUELL®-CA1 lyophilized powder through the stopper. There is more sterile diluent supplied than the 3.5 mL needed for reconstitution.

Once the sterile diluent has been added to the powder vial, remove the needle and syringe from the vial. Discard unused sterile diluent, syringe, and needle.

Gently swirl the vial until the powder is fully reconstituted into solution, leaving no visible residue or un-dissolved material.

Before each use, gently swirl to ensure a uniform solution.

Draw up the appropriate dose using a new sterile needle and syringe.

Administer the dose promptly after drawing into the dosing syringe.

Store any remaining reconstituted product at refrigerated conditions, 36˚ to 46˚F (2˚ to 8˚C). Reconstituted product remains stable under refrigeration for 28 days.
Refer to product insert for more information.
Questions?
Click below to ask a vet a question or connect to order now!
For product support call 1-800-999-0297.
1Cridge H, Lim SY, Algül H, Steiner JM. New insights into the etiology, risk factors, and pathogenesis of pancreatitis in dogs: Potential impacts on clinical practice. J Vet Intern Med. 2022 May;36(3):847-864. doi: 10.1111/



